The FIRST AND ONLY IV iron in Canada indicated in both adult and pediatric patients with IDA, as well as adult HF patients (NYHA class II/III) with ID*,1,2


CHOOSE FERINJECT
®
(ferric carboxymaltose)
Ferinject® (ferric carboxymaltose) is indicated:1
- For the treatment of iron deficiency anemia (IDA) in adult and pediatric patients 1 year of age and older when oral iron preparations are not tolerated or are ineffective.
- For the treatment of iron deficiency (ID) in adult patients with heart failure and New York Heart Association (NYHA) class II/III to improve exercise capacity.
The diagnosis of iron deficiency must be based on laboratory tests.1
IV: intravenous; IDA: iron deficiency anemia; HF: heart failure; NYHA: New York Heart Association; ID: iron deficiency.
* Comparative clinical significance has not been established.
References: 1. Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024. 2. CSL Vifor Canada DoF_Attestation Letter-A_First and Only Claim_9April2025.
Find a clinic equipped to administer Ferinject® close to your practice.
Data in ID + HF
Demonstrated Efficacy & Safety Profile Data in Chronic Heart Failure Patients (NYHA class II/III) with ID
Ferinject® significantly improved exercise capacity in CHF patients with ID compared to placebo (based on the LS mean change in the 6-MWT from baseline to Week 24: +17.5 m vs. -15.7 m; p=0.002; primary endpoint)1,2
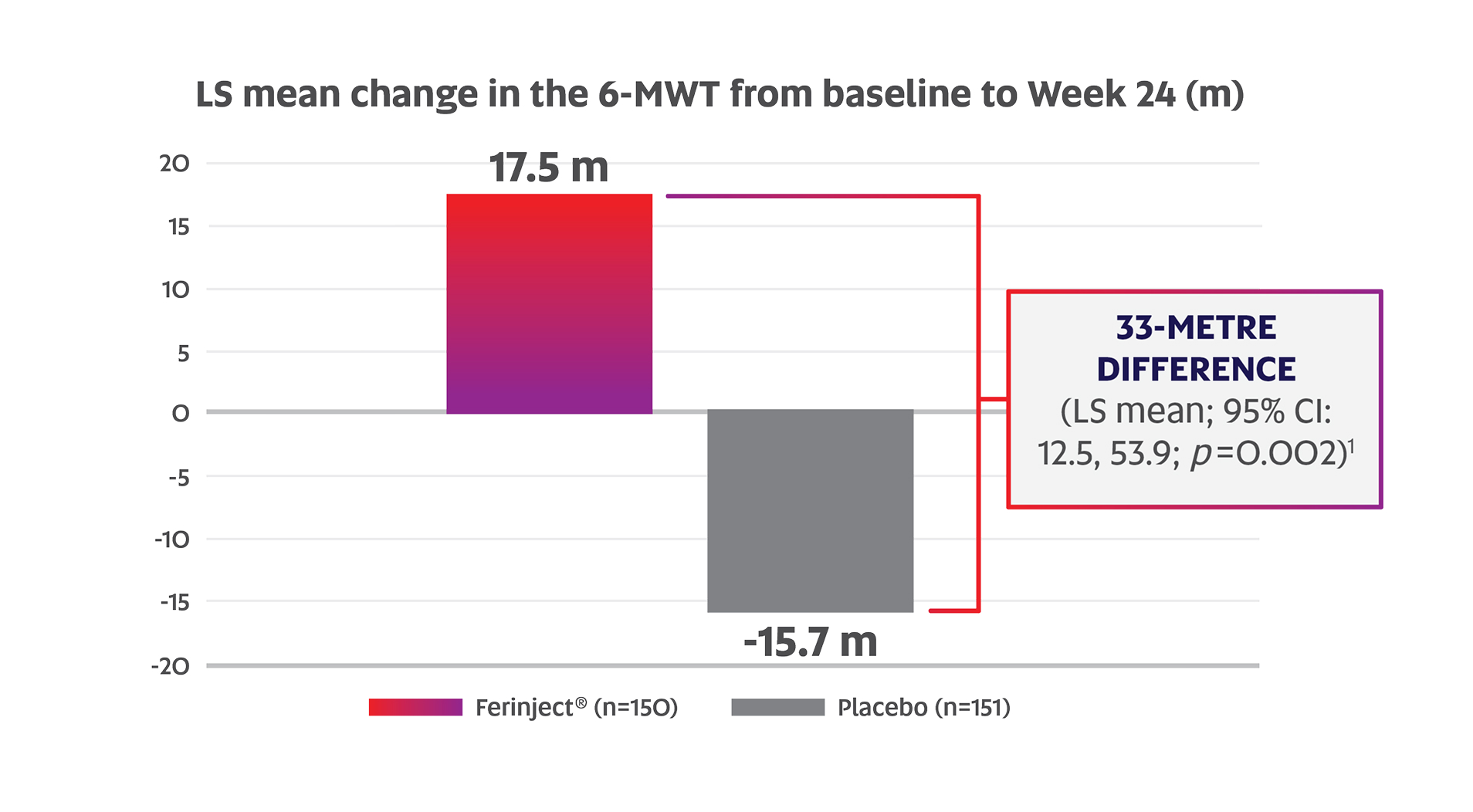
Adapted from the Ferinject® Product Monograph.1
About the 6-minute walk test (6-MWT)

The 6-MWT assesses distance walked (to the nearest metre) in 6 minutes:2
- All tests were performed along a flat, straight corridor with a hard surface, at least 25 m long with turnaround points marked by two chairs at each end of the measured course.
- Patients were instructed to walk the length of the course at their own pace while attempting to cover as much ground as possible.
- They were allowed to rest on the chairs during the test but were encouraged to resume walking as soon as they felt physically able to do so.
- Patients were advised to eat only a light meal and to avoid vigorous exercise within 2 hours prior to the test.
Adverse reactions reported in ≥2% of patients with CHF treated with Ferinject® and more frequently than placebo1
Adapted from the Ferinject® Product Monograph.1
* The group terms Sinus bradycardia, Sudden cardiac death, Vomiting, Flushing, Pain in extremity, and Dyspnea are each composed of several
near synonym terms.
† Group terms that include distinct clinical events are: Chest pain (includes Angina pectoris); Abdominal pain (Abdominal distension); Fatigue
(Malaise, Illness, Discomfort); Injection/infusion site reactions (Extravasation, Hematoma, Post procedural hematoma, Local reaction); Fracture
(Hip fracture, Rib fracture, Spinal compression fracture); Headache (Migraine, Migraine with aura); Dizziness (Vertigo, Balance disorder); Rash
(Exanthema, Urticaria, Pruritus, Boston exanthema); Hypertension (Hypertensive crisis).
Across the heart failure study (CONFIRM-HF), patients in the overall population were aged 35 to 88.1
CONFIRM-HF (cardiology study) design
A phase 4, double-blind, randomized, placebo-controlled trial to determine the effect of Ferinject® on exercise intolerance in CHF patients with ID1
Primary endpoint: The change from baseline in the 6-minute walk test (6-MWT) at 24 weeks.1
- Dosing at Day 1 and Week 6 was based on the simplified dosing table, using screening Hb and body weight.1
- Further dosing at Weeks 12, 24, and 36 was given only if serum ferritin was <100 ng/mL or 100-300 ng/mL with TSAT <20%.1
- Mean age was 68.9 in the Ferinject® group (range: 43-86) and 69.4 in the placebo group (range: 35-88).1
References & footnotes
ID: iron deficiency; HF: heart failure; NYHA: New York Heart Association; CHF: chronic heart failure; LS: least squares; 6-MWT: 6-minute walk test; CI: confidence interval; MedDRA: Medical Dictionary for Regulatory Activities; LVEF: left-ventricular ejection fraction; IV: intravenous; Hb: hemoglobin; TSAT: transferrin saturation.
‡ The study enrolled patients with mild to moderate symptoms despite optimal CHF medication and serum ferritin
<100 ng/mL (or 100 to 300 ng/mL with TSAT <20%). The mean baseline Hb was 12 g/dL. Patients with baseline Hb
≥15 g/dL were excluded.1
§ Stratified by screening Hb values.1
References
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657-668.
Data in IDA
Efficacy and Safety Profiles Were Assessed Across Multiple Therapy Areas1
Explore clinical data in:1

within 10 days after delivery
All 5 pivotal clinical trials of Ferinject® in IDA were open-label, randomized, and active-controlled. Refer to the individual page links above for additional information on the trial designs and endpoints, as well as results of each trial.1
References & footnotes
IDA: iron deficiency anemia; IBD: inflammatory bowel disease.
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Dosing & Administration
Ferinject® Dosing Follows a Stepwise Approach1
Ferinject® is for IV use only. It may be administered:1

By infusion, following dilution in sterile 0.9% sodium chloride

By injection, using undiluted dispersion

During a hemodialysis session, undiluted, directly into the venous line of the dialyser
Ferinject® must not be administered by the subcutaneous or intramuscular route.1
- The dosage of Ferinject® is expressed as mg of elemental iron:1
- 1 mL Ferinject® = 50 mg elemental iron
- Vials are available in the following compositions: 100 mg/2 mL, 500 mg/10 mL, and 1000 mg/20 mL.*,1
Find out more about:
References & footnotes
IV: intravenous.
* Vials should be visually inspected for sediment and damage before use. Use only those containing sediment-free, homogeneous dispersion.1
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Dosing & Administration
Recommended Dosing in Adult Patients
Determine the individual adult iron need1
Determine the total iron needed using the table below, taking into account your patient’s body weight and current hemoglobin (Hb) level.1
Adapted from the Ferinject® Product Monograph.1
Calculate and administer the maximum individual iron dose(s)1
Based on the total iron need determined in Step 1, administer the appropriate dose(s) of Ferinject®. Keep in mind that a single Ferinject® administration in adult patients should not exceed:1
15 mg
iron/kg
body weight
1000 mg
of iron
(20 mL Ferinject®)
Maximum recommended cumulative dose: 1000 mg of iron (20 mL Ferinject®) per week. If the total iron need is higher, each additional dose should be administered a minimum of 7 days from the last.1

HDD-CKD PATIENTS
A single maximum daily dose of 200 mg iron should not be exceeded in hemodialysis-dependent chronic kidney disease patients.1

PREGNANT WOMEN
Treatment should be confined to gestation Week 16 and beyond if the benefit is judged to outweigh the potential risk to both the mother and fetus. The maximum cumulative dose is restricted to 1000 mg if Hb >90 g/L and 1500 mg if Hb ≤90 g/L. Do not administer more than 1000 mg iron per week.1
Reassess post-iron repletion1
Reassessment should be performed by the clinician based on the individual patient’s condition.1
Reassess hemoglobin (Hb) level no earlier than 4 weeks after the final administration of Ferinject® to allow adequate time for erythropoiesis and iron utilization. If further iron repletion is required, return to Step 1.1
References & footnotes
HDD-CKD: hemodialysis-dependent chronic kidney disease.
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
About Ferinject®
An IV Iron with Worldwide Experience*,1,2
OVER
17
years in the
global market2
OVER
30
million patient-years
of experience2
Available in
87
countries2
Manufactured by
VIFOR
(International) AG2
Assessed across VARIOUS THERAPY AREAS, including in:1
The FIRST AND ONLY IV iron in Canada indicated
in all of the following:†,1,2

Adult heart failure patients (NYHA class II/III) with ID
+

Adult patients with IDA
+

Pediatric patients (ages 1+) with IDA
Mechanism of Action*
A colloidal dispersion of ferric carboxymaltose*,1
Ferinject® contains an iron complex consisting of a polynuclear iron-hydroxide core with a carbohydrate ligand.1
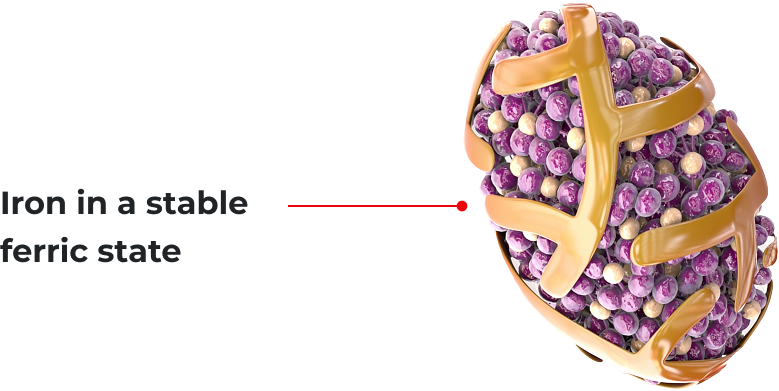
Designed to provide utilizable iron for both transport and storage*,1
Transferrin

Ferritin
Different IV iron complexes are not clinically interchangeable, as they differ in their structures, which affects their pharmacokinetic profiles.1
References & footnotes
IV: intravenous; NYHA: New York Heart Association; ID: iron deficiency; IDA: iron deficiency anemia.
* Clinical significance unknown.
† Comparative clinical significance has not been established.
References
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
- CSL Vifor Canada DoF_Attestation Letter-A_First and Only Claim_9April2025 and CSL Vifor Canada_DoF_Attestation Letter-B_Worldwide Experience Claim_2025.
Postpartum Women Data
Demonstrated Efficacy Data in Women with Postpartum IDA
Significantly more postpartum women achieved Hb >120 g/L with Ferinject® vs. oral iron between baseline and Day 42 (91.4% vs. 66.7%; p<0.0001; primary endpoint)*,1,2
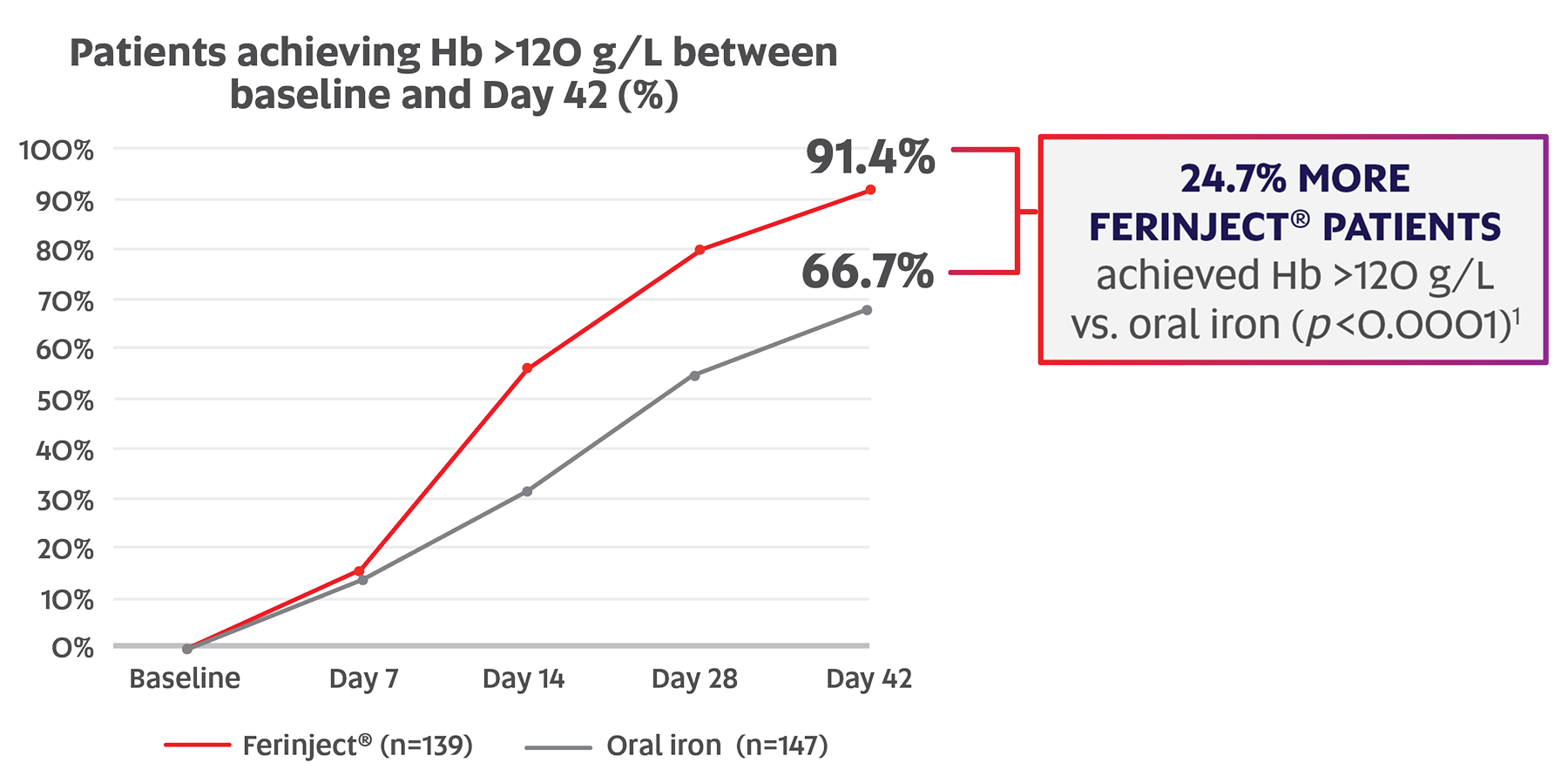
Adapted from Seid MH, et al.2
1VIT06011 (women’s health study) design
A phase 3, open-label, randomized, active-controlled trial of Ferinject® vs. oral iron1
Primary endpoint: The proportion of patients achieving Hb >120 g/L any time between baseline and the end of study (Day 42).1
Patients were randomized and stratified by:1
- Baseline Hb levels
- Screening ferritin
- Requirement for a C-section
References & footnotes
IDA: iron deficiency anemia; Hb: hemoglobin.
* The mean baseline Hb was 8.9 g/L.1
† Ferinject® dosage was based on the calculated iron deficit according to the simplified table using pre-pregnancy weight.1
References
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
- Seid MH, Derman RJ, Baker JB, et al. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obstet Gynecol. 2008;199:435.e1-7.
Data in IDA
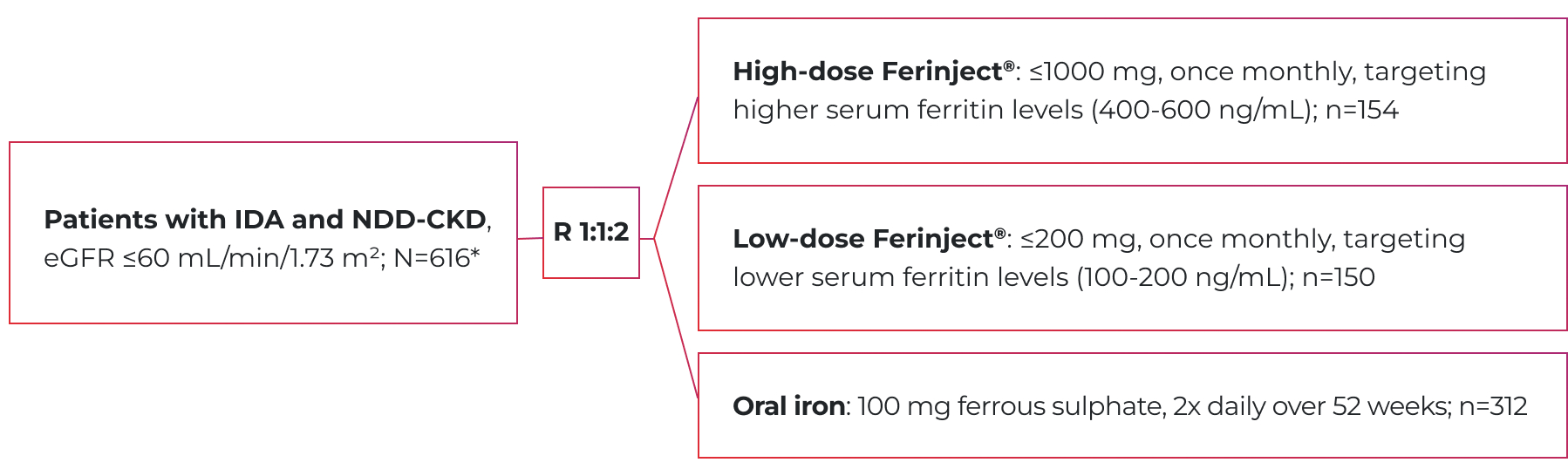
Demonstrated Efficacy Data in Patients with IDA & Non-Dialysis-Dependent CKD
High-dose Ferinject® significantly delayed the primary endpoint vs. oral iron in NDD-CKD patients (HR: 0.65; 95% CI: 0.44, 0.95; p=0.026)1,2
The primary endpoint = time to initiation of additional or alternative anemia management or two consecutive Hb values <10 g/L (without an Hb increase of ≥5 g/L between the values).1
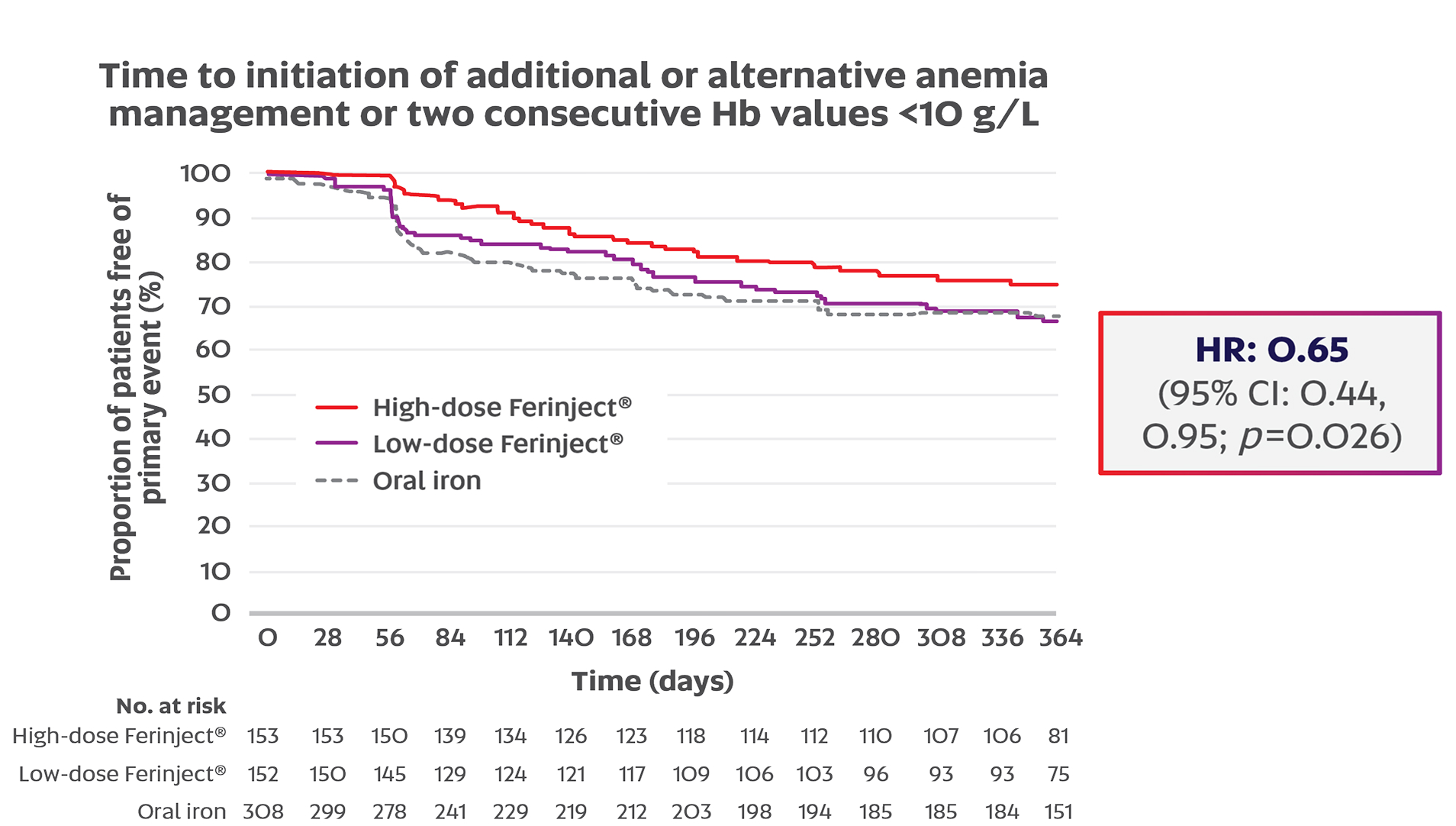
Adapted from Macdougall IC, et al.2
FIND-CKD (nephrology study) design
A phase 3b, open-label, randomized, dose-ranging, active-controlled trial of Ferinject® vs. oral iron1
Primary endpoint: The time to either the initiation of additional or alternative anemia management or two consecutive Hb values <10 g/L (without an Hb increase of ≥5 g/L between the values).1
- Mean age (range) was:1
- 69.5 (23-92) for high-dose Ferinject®
- 68.1 (29-88) for low-dose Ferinject®
- 69.3 (18-96) for oral iron
- The comparison between the two Ferinject® groups was not powered to reach statistical significance.1
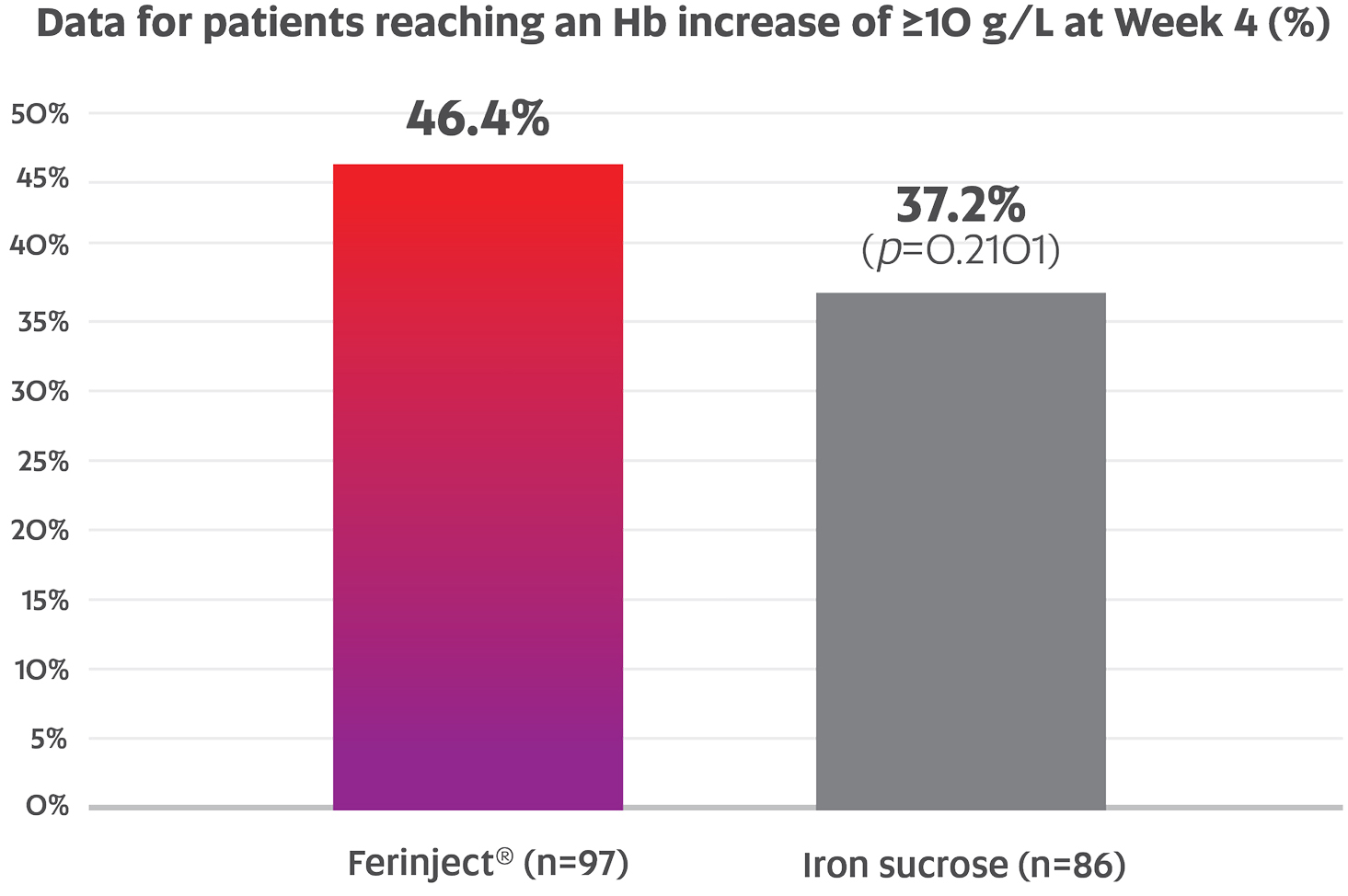
Demonstrated Efficacy Data in Hemodialysis Patients with IDA Associated with CKD
Data for proportion of patients reaching an Hb increase of ≥10 g/L at 4 weeks after baseline for Ferinject® vs. IV iron sucrose in HDD-CKD patients (46.4% vs. 37.2%; p=0.2101; primary endpoint)†,1
Adapted from the Ferinject® Product Monograph.1
Note: Data for descriptive comparative purposes only, as no formal statistical analyses were planned to directly compare Ferinject® and iron sucrose in this study.1
VIT-IV-CL-015 (nephrology study) design
A phase 3, open-label, randomized, active-controlled trial of Ferinject® vs. IV iron sucrose1
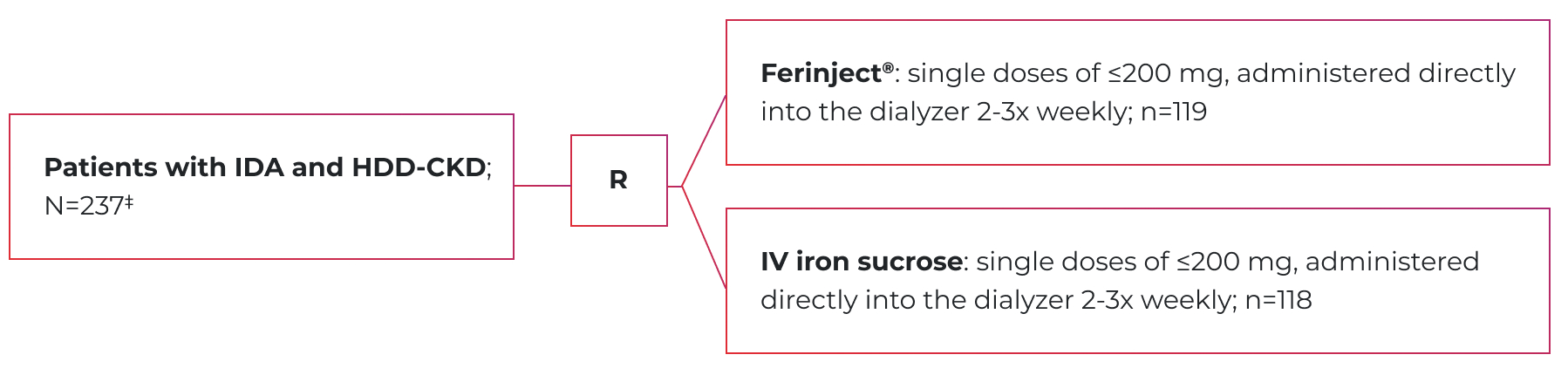
Primary endpoint: The percentage of patients in the per-protocol population reaching an Hb increase of ≥10 g/L at 4 weeks after baseline.1
- Treatment in both groups continued until the individually calculated cumulative iron dose§ was reached.1
- Mean cumulative dose of iron as Ferinject®: 1700 mg.1
- Mean age was 52.6 in the Ferinject® group (range: 22-80) and 51.0 in the IV iron sucrose group (range: 22-79).1
Note: Data for descriptive comparative purposes only, as no formal statistical analyses were planned to directly compare Ferinject® and iron sucrose in this study.1
References & footnotes
IDA: iron deficiency anemia; CKD: chronic kidney disease; NDD-CKD: non-dialysis-dependent chronic kidney disease; Hb: hemoglobin; HR: hazard ratio; CI: confidence interval; eGFR: estimated glomerular filtration rate; TSAT: transferrin saturation; IV: intravenous; HDD-CKD: hemodialysis-dependent chronic kidney disease.
* The study enrolled patients with Hb 90-110 g/L and serum ferritin <100 ng/mL or <200 ng/mL with TSAT <20%,
without erythropoiesis-stimulating agent therapy.1
† The mean baseline Hb was 93.6 g/L.1
‡ Patients had Hb ≤115 g/L and TSAT <20% or serum ferritin <200 ng/mL, with or without erythropoietin therapy.1
§ Total cumulative iron requirement was calculated using the Ganzoni formula.1
References
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
- Macdougall IC, Bock AH, Carrera F, et al. FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075-2084.
Data in IDA
Demonstrated Efficacy Data in Patients with IDA & Chronic IBD: Results vs. IV Iron Sucrose
Significantly more patients with chronic IBD responded to Ferinject® vs. IV iron sucrose by Week 12 (65.8% vs. 53.6%; p=0.004; primary endpoint)*,1
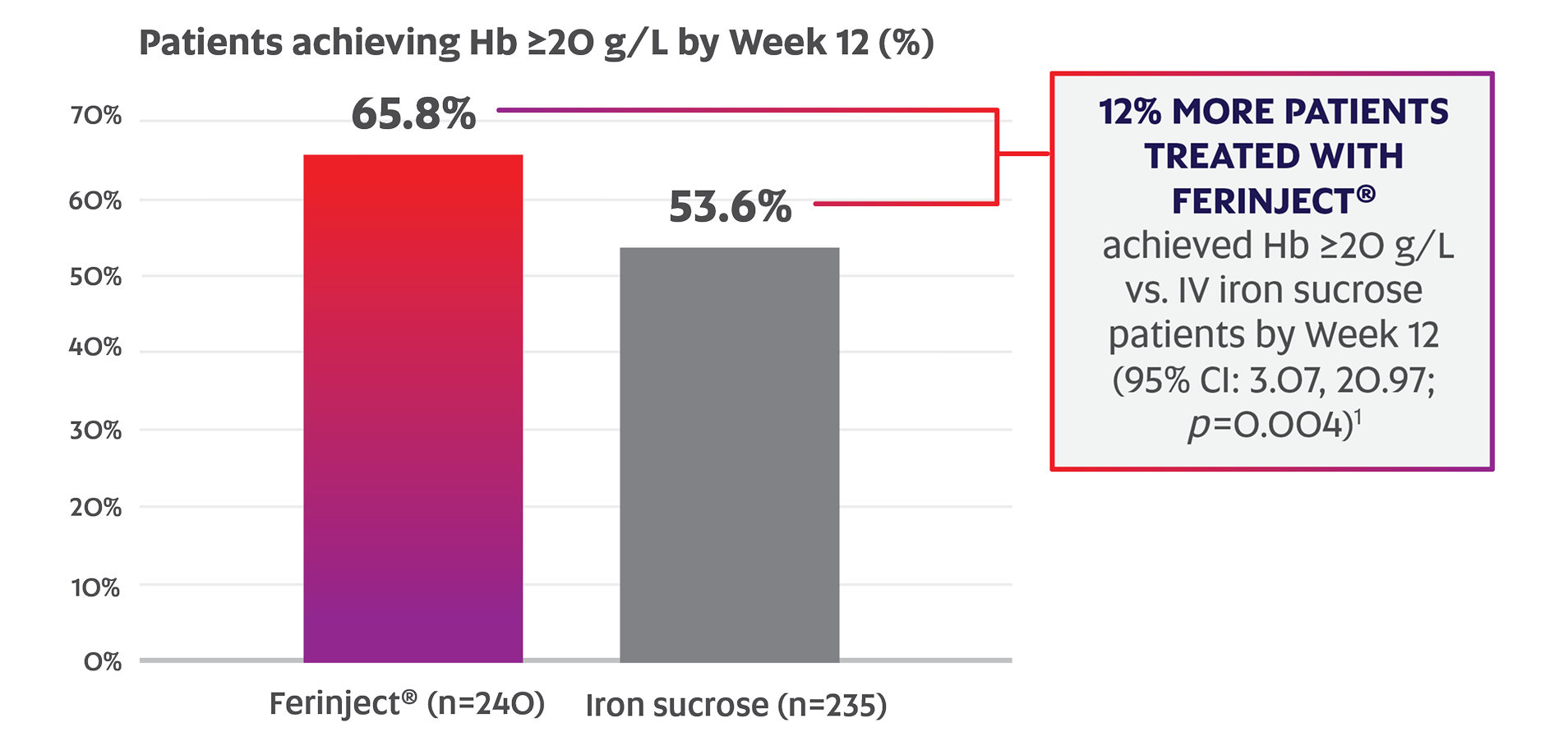
Adapted from the Ferinject® Product Monograph.1
FERGIcor (gastroenterology study) design
A phase 3, open-label, randomized, active-controlled trial of a simplified dosing schedule of Ferinject® vs. individually calculated doses of IV iron sucrose1
Primary endpoint: The number of responders, defined as Hb increase ≥20 g/L by Week 12.1
- 35.2% of Ferinject® patients had Crohn’s disease (vs. 31.0% in the IV iron sucrose group).1
- 64.8% of Ferinject® patients had ulcerative colitis (vs. 69.0% in the IV iron sucrose group).1
- Mean age was 39.7 in the Ferinject® group (range: 18-81) and 39.6 in the IV iron sucrose group (range: 18-78).1
Demonstrated Efficacy Data in Patients with IDA & Chronic IBD: Results vs. Oral Iron
Ferinject® was shown to be noninferior to oral iron in IBD patients based on mean change in Hb from baseline to Week 12 (38.3 g/L vs. 37.5 g/L; p=0.8016; primary endpoint)‡,§,1
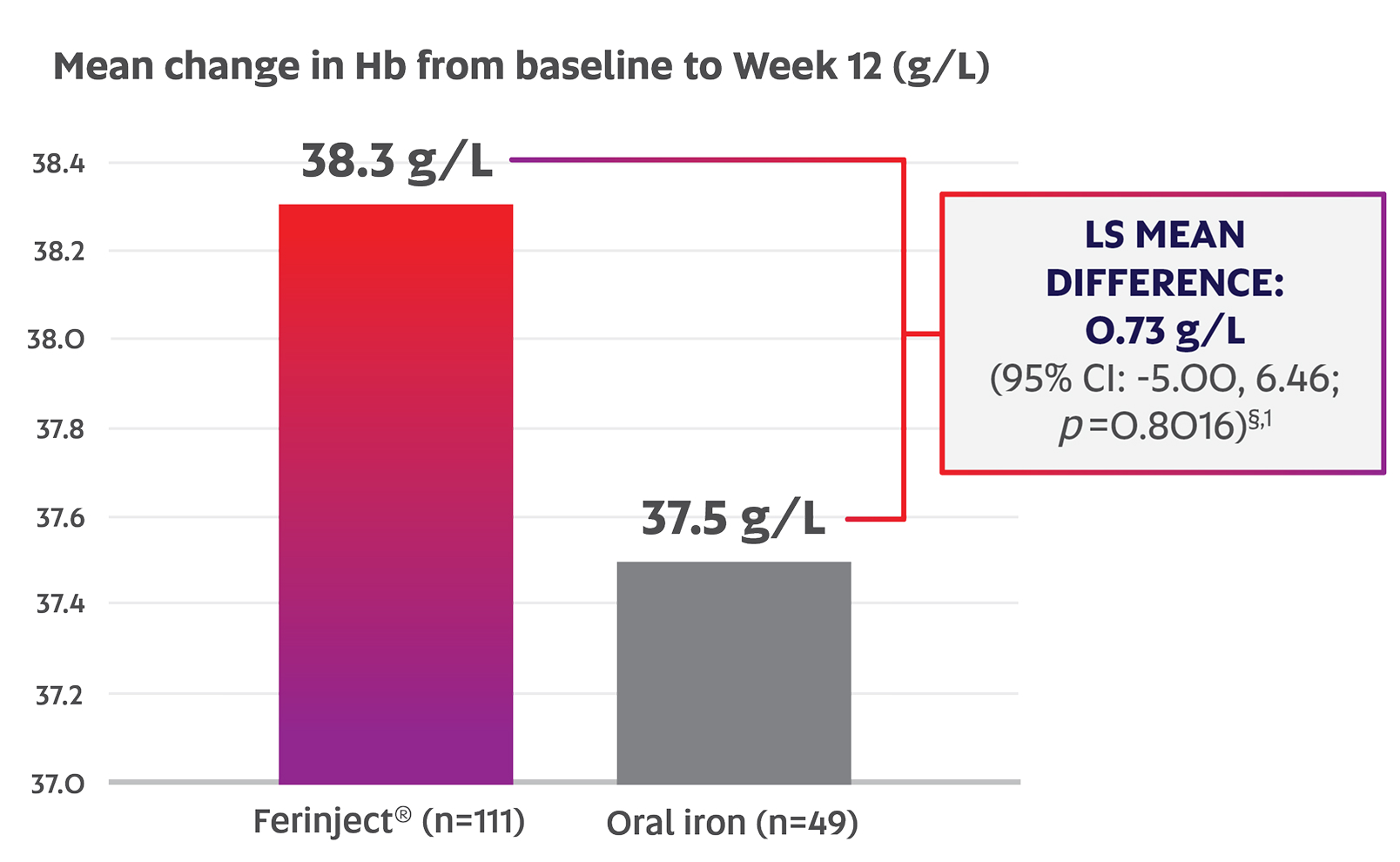
Adapted from the Ferinject® Product Monograph.1
VIT-IV-CL-008 (gastroenterology study) design
A phase 3, open-label, randomized, active-controlled, non-inferiority trial of Ferinject® vs. oral iron1
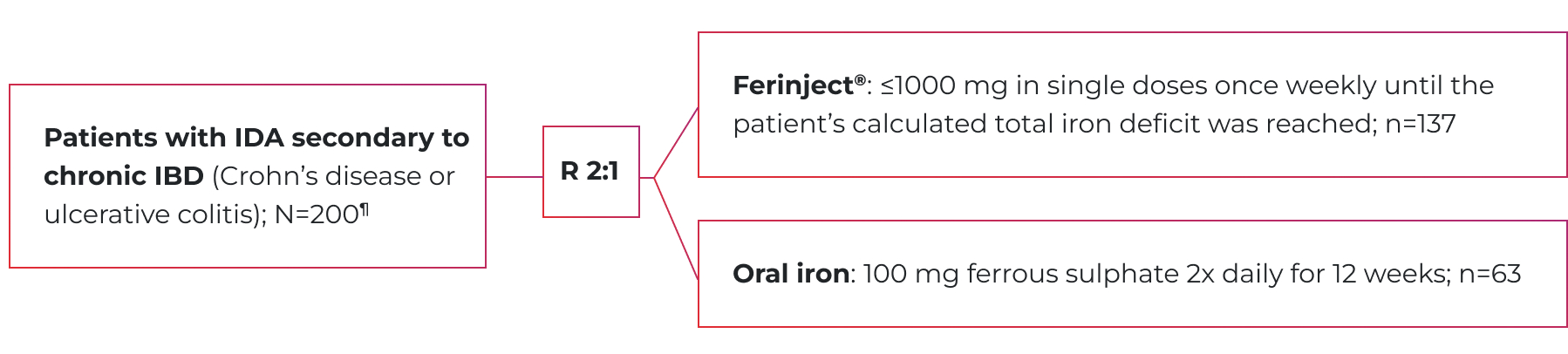
Primary endpoint: The change in Hb from baseline to Week 12.1
- 27.9% of Ferinject® patients had Crohn’s disease (vs. 26.5% in the oral iron group).1
- 72.1% of Ferinject® patients had ulcerative colitis (vs. 73.5% in the oral iron group).1
- Mean age was 40.7 in the Ferinject® group (range: 19-78) and 45.2 in the oral iron group (range: 20-78).1
References & footnotes
IDA: iron deficiency anemia; IBD: inflammatory bowel disease; IV: intravenous; Hb: hemoglobin; CI: confidence interval; LS: least squares; TSAT: transferrin saturation.
* Response defined as achieving Hb ≥20 g/L by Week 12. The mean baseline Hb was 10.2 g/L.1
† Patients included in the study had Hb 70-120 g/L (women) or 70-130 g/L (men) and serum ferritin <100 ng/mL. The
iron amount required was calculated according to the Ganzoni formula.1
‡ The mean baseline Hb was 86.2 g/L.1
§ The lower limit of the 95% CI for difference of Hb changes between the treatments was -5.0 g/L; hence, non-inferiority was concluded.1
¶ Patients included in the study had baseline Hb ≤110 g/L and TSAT <20% or serum ferritin <100 ng/mL, with a
calculated iron requirement of at least 1000 mg based on the Ganzoni formula.1
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Data in IDA
Demonstrated Safety Profile in IDA
Results of the pooled safety population of 2196 adults from multiple therapy areas1
- TEAEs (all-causality): 42.4% (932 patients)
- Most commonly reported (≥2%): headache, edema, hypertension, injection/infusion site reactions, rash, arthralgia, urinary tract infections, dizziness, nausea, nasopharyngitis, and diarrhea.
- SAEs (all-causality): 6.0% (131 patients)
- No SAEs were reported in >1% of patients treated with Ferinject®.
- Pyrexia, headache, and pulmonary embolism, reported in 1 patient each, were the only treatment-related SAEs reported for Ferinject® patients.
- Discontinuation due to TEAEs: 0.3% (6 TEAEs)
- There were 0.5% TEAEs leading to death, none of which was considered related to Ferinject®.
Adverse reactions reported in ≥1% of adult patients treated with Ferinject®1
Adapted from the Ferinject® Product Monograph.1
MedDRA: Medical Dictionary for Regulatory Activities; IV: intravenous.
* The group terms Vomiting, Pyrexia, and Dyspnea are each composed of several near synonym terms.
† Group terms that include distinct clinical events are: Chest pain (includes Angina pectoris); Abdominal pain (Abdominal distension); Edema (Peripheral
swelling); Injection/infusion site reactions (Extravasation, Hematoma, Post procedural hematoma, Local reaction); Fatigue (Malaise, Illness, Discomfort);
Upper respiratory tract infection (Rhinitis, Sinusitis); Lower respiratory tract infection (Bronchitis, Pneumonia); Influenza (Influenza-like illness); Arthralgia
(Joint stiffness, Joint swelling); Muscle spasms (Musculoskeletal stiffness); Headache (Migraine, Migraine with aura); Dizziness (Vertigo, Balance disorder);
Rash (Exanthema, Urticaria, Pruritus, Boston exanthema); Hypertension (Hypertensive crisis); Hypotension (Blood pressure abnormal, Dialysis hypotension).
Comparable frequency of TEAEs in pediatric patients (aged 1-17 years) as in the overall study population‡,1
- TEAEs (all causality): 35%
- Most commonly reported (≥5%): hypophosphatemia, rash, injection/infusion site reaction, headache, and vomiting.
- No SAEs among pediatric patients
- 1 TEAE leading to discontinuation (injection site pain)
- No new or unexpected TEAEs were observed in the pediatric population compared to those reported in the adult population.
Adverse reactions reported in ≥1% of pediatric patients treated with Ferinject®1
Adapted from the Ferinject® Product Monograph.1
IDA: iron deficiency anemia; MedDRA: Medical Dictionary for Regulatory Activities.
§ The group terms Vomiting and Flushing are each composed of several near synonym terms.
¶ Group terms that include distinct clinical events are: Injection/infusion site reactions (includes Extravasation,
Hematoma, Post procedural hematoma, Local reaction); Headache (Migraine, Migraine with aura); Rash
(Exanthema, Urticaria, Pruritus, Boston exanthema).
References & footnotes
IDA: iron deficiency anemia; TEAE(s): treatment-emergent adverse event(s); SAEs: serious TEAEs.
‡ The safety of Ferinject® in pediatric patients with IDA was evaluated in a randomized, active-controlled study of
78 patients aged 1 to 17 years (median 14.5 years). Forty patients received Ferinject® 15 mg/kg to a maximum single
dose of 750 mg on Days 0 and 7 for a maximum total dose of 1500 mg, while 38 patients received oral ferrous sulfate
for 28 days.1
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Dosing & Administration
Instructions for Intravenous (IV) Administration

Recommendations for administration by IV infusion1
When administering Ferinject® by IV infusion, it must be diluted in sterile 0.9% sodium chloride solution and administered following the recommendations below:1
Adapted from the Ferinject® Product Monograph.1
- Ferinject® must only be mixed with sterile 0.9% sodium chloride solution. No other IV dilution solutions or therapeutic agents should be used, as there is the potential for precipitation and/or incompatibility.1
- Diluted Ferinject® for IV infusion should be used within 24 hours of dilution when stored at 2 to 8°C.1
Ferinject® should not be diluted to concentrations less than 2 mg iron/mL (not including the volume of the Ferinject® dispersion).1
Recommendations for administration by IV injection1
When administering Ferinject® by IV injection using undiluted dispersion, follow the recommended rates of administration in the table below:1
Adapted from the Ferinject® Product Monograph.1
Undiluted Ferinject® for IV injection should be used immediately after opening.1
Ferinject® should only be administered when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions. Patients should be observed for signs and symptoms of hypersensitivity reactions, including monitoring of blood pressure and pulse, during and for ≥30 minutes following each administration of Ferinject®.1
For complete dosing and administration information, please refer to the Ferinject® Product Monograph.
References & footnotes
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Dosing & Administration

Recommended Dosing in Pediatric Patients
Determine the individual pediatric iron need1
After confirming iron deficiency by laboratory tests, determine the individual iron need based on the patient’s body weight and hemoglobin (Hb) level. The individual total iron need must be calculated for each patient either with the Ganzoni formula or according to the table below.1
Ganzoni formula
Calculate the iron need, rounded to the nearest 50 mg, using the Ganzoni formula:1
Total iron deficit [mg] =
body weight [kg] x (target Hb-actual Hb) [g/dL] x 2.4 + storage iron [mg]
- For patients weighing <35 kg: calculate using 13 g/dL for the target Hb and 15 mg/kg for the storage iron.1
- For patients weighing ≥35 kg: calculate using 15 g/dL for the target Hb and 500 mg for the storage iron.1
Table for determining the total iron need
Adapted from the Ferinject® Product Monograph.1
Regardless of the option used to calculate the total iron needed, it must be rounded to the nearest 50 mg. The cumulative dose should not exceed those calculated by the Ganzoni formula or shown in the table above.1
Calculate and administer the maximum individual iron dose(s)1
Based on the total iron need determined in Step 1, administer the appropriate dose(s) of Ferinject®. Keep in mind that a single Ferinject® administration in pediatric patients should not exceed:1
15 mg
iron/kg
body weight
750 mg
of iron
(15 mL Ferinject®)
Maximum recommended cumulative dose: 750 mg of iron (15 mL Ferinject®) per week. If the total iron need is higher, each additional dose should be administered a minimum of 7 days from the last.1

HDD-CKD PATIENTS
The efficacy and safety of Ferinject® have not been investigated in children and adolescents with chronic kidney disease requiring hemodialysis. Ferinject® is therefore not recommended for use in this population.1
Reassess post-iron repletion1
Reassessment should be performed by the clinician based on the individual patient’s condition.1
Reassess hemoglobin (Hb) level no earlier than 4 weeks after the final administration of Ferinject® to allow adequate time for erythropoiesis and iron utilization. If further iron repletion is required, the iron need should be recalculated using the Ganzoni formula in Step 1.1
References & footnotes
HDD-CKD: hemodialysis-dependent chronic kidney disease.
Reference
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Support & Resources
Helpful Downloads for You & Your Patients


Guide for Healthcare Providers (HCPs)
A step-by-step guide for Canadian prescribers and other members of the healthcare team that covers the dosing and administration of Ferinject®, along with Important Safety Information.
Download PDF
Ferinject® Product Monograph
Comprehensive information for healthcare providers and patients.
Download PDFWondering where to send your patients for their Ferinject® dose?
Support & Resources
Clinic Locator
Ready to prescribe Ferinject®? Find an infusion clinic near you/your patient by entering a postal code below.
Find an infusion clinic near you
Search for clinics in your area and view them on an interactive map.
Access Healthcare Services
2625 Queensview Dr, suit 105
Ottawa
Ontario
K2B 8K2
Insurance: accesshealthcaresevices
Bayshore Infusion Clinic - Calgary (South)
10233 Elbow Drive SW, Unit 125
Calgary
Alberta
T2W 1E8
Insurance: bayshore
Bayshore Infusion Clinic - Edmonton
10230 142nd Street NW, Suite 205
Edmonton
Alberta
T5N 3Y6
Insurance: bayshore
Bayshore - Fort McMurray
8106 Fraser Ave, Suite 55
Fort McMurray
Alberta
T9H 0G1
Bayshore Infusion Clinic - Grande Prairie
10104 97 Ave, Suite 201
Grande Prairie
Alberta
T8V 7X6
Bayshore - Medicine Hat
35 7th Street SE
Medicine Hat
Alberta
T1A 1J2
Bayshore Infusion Clinic - Red Deer
5201 43 Street
Red Deer
Alberta
T4N 1C7
Bayshore Infusion Clinic - Abbotsford
2051 McCallum Road
Abbotsford
British Colombia
V2S 3N5
Bayshore Infusion Clinic - Kelowna
3001 Tutt Street, Suite 210
Kelowna
British Colombia
V1Y 2H4
Bayshore Infusion Clinic - New Westminster
301 East Columbia Street, Suite 104
New Westminster
British Colombia
V3L 3W5
Bayshore Infusion Clinic - North Vancouver
135 East 15th Street
North Vancouver
British Colombia
V7L 2P7
Bayshore Infusion Clinic - Prince George
1100 Alward St
Prince George
British Colombia
V2M 7B1
Bayshore Infusion Clinic - Richmond
6051 Gilbert Road, Suite 301
Richmond
British Colombia
V7C 3V3
Bayshore Infusion Clinic - Surrey
13710 94A Avenue, Suite 307
Surrey
British Colombia
V3V 1N1
Bayshore Infusion Clinic - Vancouver
750 West Broadway
Vancouver
British Colombia
V5Z 1K1
Bayshore Infusion Clinic - Vernon
3310 32 Ave
Vernon
British Colombia
V1T 2M6
Bayshore Infusion Clinic - Victoria
1831 Oak Bay Ave
Victoria
British Colombia
V8R 1C3
Bayshore Infusion Clinic - Winnipeg
2265 Pembina Highway, Markham Professional Centre, Suite 207
Winnipeg
Manitoba
R3T 5J3
Bayshore Infusion Clinic - Fredericton
1015 Regent Street, Suite 500
Fredericton
New Brunswick
E3B 6H5
Bayshore Infusion Clinic - Saint John
560 Main St
Saint John
New Brunswick
E2K 1J5
Bayshore - Woodstock
100 Jones Street, Suite 102
Woodstock
New Brunswick
E7M 0H6
Bayshore - Bathurst ICN
515 Youghall Drive
Bathurst
New Brunswick
E2A 4X7
Bayshore - Moncton ICN
1116 Mountain Road
Moncton
New Brunswick
E1C 2T8
Bayshore Infusion Clinic - St. John's
9 Paton Street, Unit A
St.Johns
New Foundland and Labradour
A1B 4S8
Bayshore Infusion Clinic - Sydney
45 Weatherbee Road, Suite 403B Sydney Health Park
Sydney
Nova Scotia
B1M 0A1
Bayshore - Halifax ICN
7071 Bayers Rd
Halifax
Nova Scotia
B3L 2C2
Bayshore Infusion Clinic - Barrie
480 Huronia Road
Barrie
Ontario
L4N 6M2
Bayshore Infusion Clinic - Brampton
195 County Court Blvd
Brampton
Ontario
L6W 4P7
Bayshore Infusion Clinic - Guelph
89 Dawson Road, Guelph Medical Place, 1st floor
Guelph
Ontario
N1H 1B1
Bayshore Infusion Clinic - Hamilton
849 Upper Wentworth Street, Wentworth Limeridge Medical Centre, Suite 206B
Hamilton
Ontario
L9A 5H4
Bayshore Infusion Clinic - Kingston
724L Arlington Park Place
Kingston
Ontario
K7M 8H9
Bayshore Infusion Clinic - London
595 Bradley Avenue, Bradley Medical Centre, 2nd Floor
London
Ontario
N6E 3Z8
Bayshore Infusion Clinic - Markham
7800 Kennedy Rd.
Markham
Ontario
L3R 2C8
Bayshore Infusion Clinic - Toronto (Yonge)
4950 Yonge Street, Suite 908
North York
Ontario
M4S 6K1
Bayshore Infusion Clinic - Oakville
2525 Old Bronte Road, Palermo Professional Centre, Suite 210
Oakville
Ontario
L6M 4J2
Bayshore Infusion Clinic - Ottawa
117 Centrepointe Drive, Centrepointe Commerce Court, Suite 210
Ottawa
Ontario
K2G 5X3
Bayshore Infusion Clinic - Richmond Hill
9325 Yonge Street, South Hill Shopping Centre, Suite 8
Oakville
Ontario
L4C 0A8
Bayshore - Scarborough
520 Ellesmere Road, Suite 213
Scarborough
Ontario
M1R 0B1
Bayshore Infusion Clinic - Sudbury
2120 Regent Street
Sudbury
Ontario
P3E 3Z9
Bayshore Infusion Clinic - Timmins
119 Pine Street S, Suite 214
Timmins
Ontario
P4N 2K4
Bayshore Infusion Clinic - Toronto (Bloor)
2425 Bloor Street W, Toronto West Professional Centre, Suite 215
Toronto
Ontario
M6S 4W4
Bayshore Infusion Clinic - Windsor
700 Tecumseh Rd E, Windsor Health Centre, Suite 305
Windsor
Ontario
N8X 4T2
Bayshore - Burlington ICN
5045 Mainway
Burlington
Ontario
L7L 5Z1
Bayshore - Chatham ICN
20 Emma Street
Chatham
Ontario
N7L 5K5
Bayshore - Newmarket ICN
679 Davis Drive
Newmarket
Ontario
L3Y 5G8
Bayshore - Niagara Falls ICN
6453 Morrison St
Niagara Falls
Ontario
L2E 2G5
Bayshore - Orillia ICN
210 Memorial Ave
Orillia
Ontario
L3V 7V1
Bayshore - Owen Sound ICN
250 10th Street East
Owen Sound
Ontario
N4K 1S4
Bayshore - Sarnia ICN
265 Front Street North
Sarnia
Ontario
N7T 5S6
Bayshore - Toronto Downtown ICN
790 Bay Street
Toronto
Ontario
M5G 1N8
Bayshore - Vaughan ICN
8333 Weston Rd
Woodbridge
Ontario
L4L8E2
Bayshore Infusion Clinic - Brossard
1100 rue du Lux
Brossard
Qubec
J4Y 0E2
Bayshore - Chicoutimi ICN
255 rue racine est
Chicoutimi
Qubec
G7H 7L2
Clinique de perfusion Bayshore - Laval
3300, ave 100 Place Laval 440
Laval
Qubec
H7T 0J7
Clinique de perfusion Bayshore - Montréal (St. Antoine)
450, rue Saint Antoine E, Clinique Médicale Agatha, Suite 510
Montréal
Qubec
H2Y 1A5
Clinique de perfusion Bayshore - Montréal
5100 de Maisonneuve Ouest, Suite 601
Montréal
Qubec
H4A 3T2
Clinique de perfusion Bayshore - Pointe-Claire
955, boulevard St-Jean, Suite 214 (Centre Médical Brunswick)
Pointe-Claire
Qubec
H9R 5K3
Clinique de perfusion Bayshore - Québec City
1200, rue des Soeurs-Du-Bon-Pasteur, GMF Cité-Verte, Bloc Q, Local 220
Québec City
Qubec
G1S 0B1
Bayshore - Roberval ICN
720 boulevard Saint-Joseph
Roberval
Qubec
G8H 2L2
Clinique de perfusion Bayshore - Sherbrooke
360 Galt E, suite 104
Sherbrooke
Qubec
J1G 1X9
Clinique de perfusion Bayshore - Trois-Rivières
1785, boulevard du Carmel, Suite 101B
Trois-Rivières
Qubec
G8Z 3R8
Bayshore - Ste Julie ICN
2105 Armand- Frappier
Ste Julie
Qubec
J3E 2N7
Bayshore - Victoriaville ICN
39 rue Laurier Est
Victoriaville
Qubec
G6P 6P6
Bayshore Infusion Clinic - Regina
2125 11th Ave
Regina
Saskatchewan
S4P 3X3
Coverdale Clinics Medicine Hat
20 Northlands Way NE #108
Medicine Hat
Alberta
T1C 1Z2
Coverdale Clinics Lethbridge
515 7 St S Unit 101
Lethbridge
Alberta
T1J 2G8
Coverdale Clinics Edmonton Hys
Suite 206B, 11010 101 St NW
Edmonton
Alberta
T2V 4R6
Coverdale - Calgary South East
8500 Blackfoot Trail SE Unit 220
Calgary
Alberta
T2J 7E1
Coverdale - Calgary Mission
2303 4th Street SW, Suite 604
Calgary
Alberta
T2S 2S7
Coverdale - Calgary Harvest Hills
178 96 Avenue NE, Suite 213
Calgary
Alberta
T3K 6G4
Coverdale - Airdrie
401 Cooper's Boulevard SW, Suite 1109
Airdrie
Alberta
T4B 4J3
Coverdale - West Edmonton
8944 182nd Street, Suite 222
Edmonton
Alberta
T5T 2E3
Coverdale - South Edmonton
4207 98th Street NW, Suite 102, Greystone Bldg 4
Edmonton
Alberta
T6E 5R7
Coverdale - Fort McMurray
310 Thickwood Boulevard, Unit 1
Fort McMurray
Alberta
T9K 1Y1
Coverdale Clinics Kelowna
1433 St Paul St Suite 208
Kelowna
British Colombia
V1Y 2E4
Coverdale Clinics Burnaby
5050 Kingsway #507
Burnaby
British Colombia
V5H 4C2
Coverdale - Vancouver
750 West Broadway, suite 1400
Vancouver
British Colombia
V5Z 1H2
Coverdale - Richmond
13151 Vanier Place, Suite 180
Richmond
British Colombia
V6V 2J1
Coverdale - Victoria
1175 Cook St, suite 204
Victoria
British Colombia
V8V 4A1
Coverdale Clinics Brandon
2412 Victoria Ave Unit B
Brandon
Manitoba
R7B 0M5
Coverdale - Winnipeg North
109-2110 Main St
Winnipeg
Manitoba
R3G 2V2
Coverdale - Winnipeg Central
1661 Portage Ave, unit 101
Winnipeg
Manitoba
R3J 3T7
Coverdale - Winnipeg South
307-2265 Pembina Highway
Winnipeg
Manitoba
R3T 5J3
Coverdale - Steinbach
380 Stone Bridge Crossing, unit 10
Steinbach
Manitoba
R3T 5J3
Coverdale - Winkler
583 Main St, unit 4 & 5
Winkler
Manitoba
R6W 1A4
Coverdale - Moncton Providence
27 rue Providence, Suite 148
Moncton
New Brunswick
E1C 8X4
Coverdale - Miramichi
250 Pleasant Street, Suite 8
Miramichi
New Brunswick
E1V 1Y5
Coverdale - Tracadie
3980 Rue Principal, Unit 1A
Tracadie-Sheila
New Brunswick
E1X 1B6
Coverdale - Saint John
703 Millidge Ave
Saint John
New Brunswick
E2K 2N7
Coverdale - Fredericton
1015 Regent St, suite 411
Fredericton
New Brunswick
E3B 6H5
Coverdale - St Stephen
89 Prince William St, suite A
St. Stephen
New Brunswick
E3L 1S8
Coverdale - Campbellton
10 Village Ave, suite 16
Campbellton
New Brunswick
E3N 3S8
Coverdale - Edmundston
66 rue Bateman, suite 1
Edmundston
New Brunswick
E3V 0E4
Coverdale Clinics Bathurst
950 Picot Ave
Bathurst
New Brunswick
E2A 4T7
Coverdale Clinics Mount Pearl
30 Mt Carson Ave
Mount Pearl
New Foundland and Labradour
A1N 3K4
Coverdale - St.Johns Stavanger
120 Stavanger Drive, Suite 101
St.Johns
New Foundland and Labradour
A1A 5E8
Coverdale - Gander
60 Memorial Drive
Gander
New Foundland and Labradour
A1V 1C9
Coverdale - Grand Falls-Windsor
28 Cromer Avenue
Grand Falls-Windsor
New Foundland and Labradour
A2A 1X2
Coverdale - Corner Brook
2 Herald Ave, suite 214
Corner Brook
New Foundland and Labradour
A2H 4B5
Coverdale Clinics Sydney River
31 Riverside Dr
Sydney
Nova Scotia
B1S 3N1
Coverdale - Sydney
45 Weatherbee Rd, suite 306A
Sydney
Nova Scotia
B1M 0A1
Coverdale - Antigonish
40 Church St
Antigonish
Nova Scotia
B2G 2C7
Coverdale Clinics Halifax
5991 Spring Garden Rd suite 480
Halifax
Nova Scotia
B3H 1Y6
Coverdale - New Minas
8799 Commercial St
New Minas
Nova Scotia
B4N 3C4
Coverdale Clinics Spryfield
Herring Cove Rd
Halifax
Nova Scotia
B3R 1V9
Coverdale Clinics Bridgewater
Glen Allan Dr
Bridgewater
Nova Scotia
B4V 3N2
Coverdale Clinics Yarmouth
345 Hwy 1 Suite 2A, Dayton
Dayton
Nova Scotia
B5A 5A1
Coverdale Clinics Ottawa East
1900 City Park Dr,
Gloucester
Ontario
K1J 1A3
Coverdale - Ottawa
2039 Robertson Road, unit 505
Ottawa (Nepean)
Ontario
K2H 8R2
Coverdale - Cornwall
171 Montreal Rd, suite 202
Cornwall
Ontario
K6H 1B2
Coverdale - Belleville
210 Dundas St, suite 205
Belleville
Ontario
K8N 5G8
Coverdale - Peterborough
270 Charlotte St, suite 101
Peterborough
Ontario
K9J 2V4
Coverdale - St. Catharines
245 Pelham Road, Suite 101
St. Catharines
Ontario
L2S 1X8
Coverdale - Orillia
119 Memorial Ave, suite 102
Orillia
Ontario
L3V 5X1
Coverdale - Barrie
1 Quarry Ridge Rd, suite LL1
Barrie
Ontario
L4M 7G1
Coverdale - Mississauga
3420 Hurontario St, suite 202
Mississauga
Ontario
L5B 4A9
Coverdale - Oakville
1393 North Service Rd E, unit 1
Oakville
Ontario
L6H 1A7
Coverdale - Burlington
672 Brant St, suite 401
Burlington
Ontario
L7R 2H3
Coverdale - Hamilton
460 Main Street East, Suite 303
Hamilton
Ontario
L8N 1K4
Coverdale - Scarborough
1371 Neilson Rd, suite 323
Scarborough
Ontario
M1B 4Z8
Coverdale - Toronto NE Allergy
240 Duncan Mill Road
Toronto
Ontario
M3B 3S6
Coverdale - Toronto East - Don Mills
20 Wynford Dr, suite 301
North York
Ontario
M3C 1J4
Coverdale - North York
2065 Finch Ave W, suite 209, 2nd floor
North York
Ontario
M3N 2V7
Coverdale - Toronto Downtown
123 Edward St, Suite 914
Toronto
Ontario
M5G 1E2
Coverdale - Downtown Guelph
21 Surrey St. W
Guelph
Ontario
N1H 3R3
Coverdale Clinics Brampton
Suite 305, 36 Vodden St E
Brampton
Ontario
L6V 4H4
Coverdale - Kitchener-Waterloo
100 Highland Road West, Suite 4
Kitchener
Ontario
N2M 3B5
Coverdale - Woodstock
600 Princess St, suite 302
Woodstock
Ontario
N4S 4H4
Coverdale - London
339 Wellington Road, unit 140
London
Ontario
N6B 5Z9
Coverdale - Windsor Allergy Clinic
1407 Ottawa Street
Windsor
Ontario
N8X 2G1
Coverdale - Windsor
700 Tecumseh Road East, Suite 300
Windsor
Ontario
N8X 4T2
Coverdale - North Bay
1500 Fisher St., Unit 208b
North Bay
Ontario
P1B 2H3
Coverdale - Bracebridge
345 Ecclestone Dr, suite 1053
Bracebridge
Ontario
P1L 1R1
Coverdale - Sudbury
2141 Lasalle Blvd.
Sudbury
Ontario
P3A 2A3
Coverdale - Sault Ste Marie
170 East St, Suite 104
Sault Ste. Marie
Ontario
P6A 3C6
Coverdale - Charlottetown
199 Grafton Street, Suite 303
Charlottetown
Prince Edwards Island
C1A 1L2
Coverdale - Grande-Rivière
200 Grande-Allée Est
Grande-Rivière
Qubec
G0C 1K0
Coverdale - Rivière du Loup
240 rue Lafontaine, suite 102
Rivière-du-Loup
Qubec
G5R 3A7
Coverdale - Trois-Rivières Est
1117 rue Sainte Marguerite
Trois-Rivières
Qubec
G8Z 1Y2
Coverdale - Trois-Rivières
1900 boul des Recollets, suite 120A
Trois-Rivières
Qubec
G8Z 4K4
Coverdale - Montreal West
260 Dunbar Avenue, Suite 100
Mont-Royal
Qubec
H3P 2H5
Coverdale Clinics Jonquiere
3750 Royaume Blvd #200
Jonquière
Qubec
G7X 0A4
Coverdale - Laval
1695 Boul. Laval, Suite 325
Laval
Qubec
H7S 2M2
Coverdale - Contrecoeur
4915 route Marie-Victorin, suite 206
Contrecoeur
Qubec
J0L 1C0
Coverdale Clinics Montreal Centrale
535 Ontario St E
Montreal
Qubec
H1N 1C1
Coverdale - Drummondville
2125 Boul Lemire, Suite 140
Drummondville
Qubec
J2B 8N8
Coverdale - Saint Hyacinthe
2780 Ave Raymond, suite 208
Saint Hyacinthe
Qubec
J2S 5W7
Coverdale - Greenfield Park
4898 Taschereau Boulevard, Suite 101
Greenfield Park
Qubec
J4V 2J2
Coverdale Clinics NDG (Notre-Dame-De-Grace)
5025 Sherbrooke St W
Westmount
Qubec
H4A 1S9
Coverdale - Chateauguay
230 Boul Brisebois, suite 102
Chateauguay
Qubec
J6K 4Y6
Coverdale Clinics Laval Carrefour
3030 Boul. le Carrefour #304
Laval
Qubec
H7T 2P5
Coverdale Clinics Saint Lazare
Suite 210, 1965 Chemin Ste Angélique
Saint-Lazare
Qubec
J7T 0E2
Coverdale - Gatineau
520 Boulevard De l'hôpital, Suite 3C
Gatineau
Qubec
J8V 2P5
Coverdale - Rouyn-Noranda
243 Avenue Murdoch, Suite 106
Rouyn-Noranda
Qubec
J9X 1E8
Coverdale - Regina
1621 Albert St, suite 136
Regina
Saskatchewan
S4P 2S5
Coverdale - Swift Current
244 1st Ave NE, unit 8
Swift Current
Saskatchewan
S9H 2B4
The Catalyst Centre – Oshawa
Taunton Surgical Centre, 1300 Keith Ross Drive
Oshawa
Ontario
L1J 0C7
The Catalyst Centre – Whitby
Whitby Health Centre, 198 Des Newman Drive
Whitby
Ontario
L1P 0P9
Riverside South Medica Centre
665 Earl Armstrong Rd #3
Gloucester
Ontario
K1V 1T4
Charlton Centre Hamilton & Stoney Creek MAIN
Unit # 4, 211 Pritchard Rd
Hamilton
Ontario
L8J 0G5
Charlton Centre Brampton
Suite 301 - 2250 Bovaird Dr E
Brampton
Ontario
L6R 0W3
Charlton Centre Barrie
Suite 300, 15 Gallie Court
Brrie
Ontario
L4M 7G1
Charlton Centre Brantford
D - 17 Corporate Place
Brantford
Ontario
N3R 8A6
Charlton Centre Cambridge
Suite 201 - 745 Coronation Blvd
Cambridge
Ontario
N1R 0B6
Charlton Centre Hamilton Downtown
Suite 403 - 25 Charlton Ave East
Hamilton
Ontario
L8N 1Y2
Charlton Centre Huntsville
Suite 205 - 348 Muskoka Road 3 North
Huntsville
Ontario
P1H 1H8
Charlton Centre Kitchener
Suite 201 - 564 Belmont Ave West
Kitchener
Ontario
N2M 5N6
Charlton Centre Orillia
79 Colborne St East
Orillia
Ontario
L3V 1T6
Charlton Centre Richmond Hill
20 Vogell Road, unit C
Richmond Hill
Ontario
L4B 3L1
Charlton Centre Scarborough
Suite 920-305 Milner Avenue
Scarborough
Ontario
M1B 3V4
Charlton Centre St. Catharines
Suite 5-2 Lakeshore Road
St. Catharines
Ontario
L2N 7E4
Charlton Centre Stoney Creek (part of Hamilton Main)
Unit # 4, 211 Pritchard Rd
Stoney Creek Mtn
Ontario
L8J 0G5
Charlton Centre Sudbury
1935 Paris Street
Sudbury
Ontario
P3E 6C3
Charlton Centre Tecumseh
Suite 20 - 13278 Tecumseh Road East
Tecumseh
Ontario
N8N 3T6
Charlton Centre Timmins
Suite 205, 707 Ross Ave East
Timmins
Ontario
P4N 8R1
Charlton Centre Toronto (Advanced@Charlton)
Suite 105, 700 University Avenue
Toronto
Ontario
M5G 1X6
Charlton Centre Windsor
Suite 500 – 871 Ottawa Street
Windsor
Ontario
N8X 2C9
Dr. Kevin McLeod Inc. Infusion Clinic
101 16th St. West, Suite 200
North Vancouver
British Columbia
V7M 1T3
Care&Family Health - Lawrence Park
6010-3080 Yonge Street
Toronto
Ontario
M4N 3N1
Family Health Hub Walk-in Clinic and Family Practice
190-2525 Old Bronte Rd.
Oakville
Ontario
L6M 4J2
Pearl Family Practice Ridgeway
U-58, 3176 Ridgeway Drive, Mississauga
Mississauga
Ontario
L5L 5S6
PROXIMA Healthcare
101-320 Matheson Blvd West
Mississauga
Ontario
L5R 3R1
Rapid Access IV Iron Clinic
4789 Kingsway, Unit 525
Burnaby
British Columbia
V5H 0A3
RevIVe Iron Clinic
1100 University Ave West
Windsor
Ontario
N9A 5S7
The Gray Clinic
1053 Autumnwood Drive
Winnipeg
Manitoba
R2J 1C6
Total Life Care Medical
7155 Kingsway, Unit 250
Burnaby
British Columbia
V5E 2V1
Vital Health Pharmacy
560 West Avenue
Kelowna
British Columbia
V1Y 4Z4
Vital Health Pharmacy
1825 Fort St.,
Victoria
British Columbia
V8R 1J6
Wellspring Infusion Clinic Abbotsford
2180 Gladwin Rd., Suite 102
Abbotsford
British Columbia
V2S 0H4
Wellspring Infusion Clinic Surrey
12565 88 Avenue, Unit 106
Surrey
British Columbia
V3W 3J7
Wellspring Infusion Clinic Vancouver
943 W Broadway, Unit 510
Vancouver
British Columbia
V5Z 4E1
Stellar Health clinic- Surrey
5633 177B st.
Surrey
British Columbia
V3S 4H9
This tool is for informational purposes only. CSL Vifor is not responsible for the clinics listed nor the services provided therein. Clinic location information may not be current.
Important Safety Information
Indications and clinical use:
Ferinject® (ferric carboxymaltose) is indicated:
- for the treatment of iron deficiency anemia (IDA) in adult and pediatric patients 1 year of age and older when oral iron preparations are not tolerated or are ineffective.
- for the treatment of iron deficiency (ID) in adult patients with heart failure and New York Heart Association (NYHA) class II/III to improve exercise capacity.
The diagnosis of iron deficiency must be based on laboratory tests.
Contraindications:
Ferinject® is contraindicated in patients:
- with known serious hypersensitivity to other parenteral iron products.
- with anemia not attributed to iron deficiency (e.g., other microcytic anemia).
- with evidence of iron overload or disturbances in utilization of iron (e.g., hemochromatosis, hemosiderosis).
Serious warnings and precautions:
Hypersensitivity reactions: Ferinject® is contraindicated in patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container.
- Serious hypersensitivity reactions, including life-threatening and fatal anaphylaxis/anaphylactoid reactions, have been reported in patients receiving intravenous (IV) iron products including Ferinject®.
- Patients should be observed for signs and symptoms of hypersensitivity reactions, including monitoring of blood pressure and pulse, during and for at least 30 minutes following each administration of Ferinject®.
- Ferinject® should only be administered when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
Other relevant warnings and precautions:
- Accumulation of iron in storage sites and possible hemosiderosis from excessive therapy with parenteral iron; monitoring of hematologic response and iron parameters, such as serum ferritin and transferrin saturation (TSAT), is recommended
- Hypophosphatemia and hypophosphatemic osteomalacia; monitoring serum phosphate levels in patients with risk factors, including prior to a repeat course is recommended; monitoring is also recommended in any patient who receives a second course within three months
- Risk in patients with liver dysfunction and in patients with hepatic dysfunction where iron overload is a precipitating factor, in particular porphyria cutanea tarda (PCT)
- Hypersensitivity reactions; monitoring for signs and symptoms of a reaction during and for at least 30 minutes after administration is recommended
- Infection
- Paravenous leakage
- Limited clinical data in pregnant women; treatment should be confined to gestation week 16 and beyond if the benefit is judged to outweigh the potential risk to both the mother and fetus
- Fetal bradycardia (usually transient); monitoring of the unborn baby is recommended during administration to pregnant women
- Potential for adverse events among breastfed children of breastfeeding women treated with Ferinject®; the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Ferinject® in addition to any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition
For more information:
Please consult the Product Monograph for important information relating to adverse reactions, drug interactions, and dosing information, which has not been discussed in this piece.
The Product Monograph is also available by emailing medinfo.canada@viforpharma.com or by calling 1-866-773-7721 during business hours or 514-219-7560 for urgent or after-hours service.
References & footnotes
References
- Ferinject® Product Monograph. Vifor (International) Inc. June 27, 2024.
Adverse events should be reported. You can report any suspected side effects associated with the use of health products to Health Canada by:
- Visiting the Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax; or
- Calling toll-free at 1-866-234-2345.
Adverse events should also be reported to CSL Behring Canada (AdverseReporting@cslbehring.com).
Keep in touch
Join our mailing list for ongoing updates about Ferinject® and the resources available to you and your patients.
Sign up now